Dr. Ju-Ming Wang

Dr. Ju-Ming Wang
Position:Distinguished Professor
Group:Biomedical technology
Research Interests:Inflammation, cancer biology, gene regulation and signal transduction
E-mail:yumingw@mail.ncku.edu.tw
Room:89906
Room Tel:+886-6-2757575#58226
Laboratory Tel:+886-6-2757575#58234#910

| School | Department | Country | Degree | Period |
| National Defense Medical Center | Institute of Life Science | TW | Ph.D | 1995.08 ~ 1999.12 |
| National Taiwan Ocean University | Institute of Biotechnology | TW | M.S. | 1993.07 ~ 1995.07 |
| Fu-Jen Catholic University | Department of Biology | TW | B.S. | 1989.09 ~ 1993.06 |

|
Institute |
Position |
Period |
|
National Cheng Kung University |
Distinguished Professor |
2016.08~now |
|
College of Bioscience and Biotechnology |
Dean |
2022.08-now |
|
Academic Affairs, NCKU |
Vice President |
2019.02~2022.07 |
|
Academic Affairs, NCKU |
Associate Vice President |
2015.02~2019.01 |
|
Center for Teaching and Learning Development, NCKU |
Director |
2015.02~2019.01 |
|
Yunlin Chiayi and Tainan Regional Teaching Resource Center, NCKU |
Director |
2015.02~2018.01 |
|
Institute of Bioinformatics and Biosignal Transduction, NCKU |
Director |
2013.08~2017.07 |
|
Department of Biotechnology and Bioindustry Sciences |
Professor |
2016.08~now |
|
Institute of Bioinformatics and Biosignal Transduction, NCKU |
Professor |
2012.09~2017.07 |
|
Institute of Pharmacology, NCKU |
Adjunct Professor |
2012.09~now |
|
Institute of Basic Medical Sciences, NCKU |
Adjunct Professor |
2012.09~now |
|
Institute of Bioinformatics and Biosignal Transduction, NCKU |
Associate Professor |
2009.09~2012.08 |
|
Institute of Bioinformatics, NCKU |
Adjunct Assistant Professor |
2007.09~2010.08 |
|
Institute of Biosignal Transduction, NCKU |
Assistant Professor |
2006.09~2009.08 |
|
MD Anderson Cancer Center, Houston, TX, USA |
Visiting Research Scholar |
2018.06~2018.08 |
|
Department of Pharmacology, Kyoto University, JP |
Visiting Research Scholar |
2013.07~2013.09 |
|
Department of Pharmacology, Kyoto University, JP |
Visiting Research Scholar |
2012.07~2012.09 |
|
Department of Biological Chemistry, UC Irvine, CA, USA |
Visiting Research Scholar |
2011.07~2011.09 |
|
Institute of Biological Chemistry, UC Irvine, USA |
Postdoctoral |
2005.03~2006.08 |
|
Institute of Pharmacology, NCKU |
Research Assistant Professor |
2004.11~2005.02 |
|
Institute of Pharmacology, NCKU, TW |
Postdoctoral |
2003.02~2004.10 |
|
Institute of Molecular Biology, Academia Sinica |
Postdoctoral |
2000.01~2003.01 |
The causes of inflammation-related diseases (including cancer) are complex and difficult to control. How to effectively control the inflammatory response to attenuate or even solve inflammatory diseases has always been the goal of inflammation research scholars. We are committed to solving various problems caused by "abnormal inflammatory responses". My laboratory explored and clarified the molecular mechanisms of inflammation involved in the development of various diseases, we identified critical inflammatory factor targets, and developed anti-inflammatory drugs or provided more optimal therapeutic strategies for the treatment of various inflammation-related diseases. At present, through a well-established research and translational application platform combined with the basic research of molecular biology, cell biology and biochemistry, we have identified several key proteins of inflammatory responses and revealed their pathogenic mechanisms and inhibitors. A series of research technologies and materials, such as human disease animal models and gain- or loss-of function assays were conducted for provide solutions for disease treatment and evaluation of the drug effects.
.png) |
Due to the heterogeneity and high frequency of genome mutations in cancer cells, targeting vital protumour factors found in stromal cells in the tumour microenvironment may represent an ideal strategy in cancer therapy. However, the regulation and mechanisms of potential targetable therapeutic candidates need to be investigated. An in vivo study demonstrated that loss of pentraxin 3 (PTX3) in stromal cells significantly decreased the metastasis and growth of cancer cells. Clinically, our results indicate that stromal PTX3 expression correlates with adverse prognostic features and is associated with worse survival outcomes in triple-negative breast cancer (TNBC). We also found that transforming growth factor beta 1 induces PTX3 expression by activating the transcription factor CCAAT/enhancer binding protein delta in stromal fibroblasts. Following PTX3 stimulation, CD44, a PTX3 receptor, activates the downstream ERK1/2, AKT and NF-κB pathways to specifically contribute to the metastasis/invasion and stemness of TNBC MDA-MB-231 cells. Two types of PTX3 inhibitors were developed to disrupt the PTX3/CD44 interaction and they showed a significant effect on attenuating growth and restricting the metastasis/invasion of MDA-MB-231 cells, suggesting that targeting the PTX3/CD44 interaction could be a new strategy for future TNBC therapies. (Clin Transl Med. 2022; 12(1): e724.) |
.png) |
Fibrosing interstitial lung diseases (fILD) are potentially fatal with limited therapeutic options and no effective strategies to reverse fibrogenesis. Myofibroblasts are chief effector cells in fibrosis that excessively deposit collagen in the pulmonary interstitium and lead to progressive impairment of gaseous exchange. Plasma and lung specimens from patients with fILD were applied for detecting pentraxin 3 abundance by ELISA and Immunohistochemistry. Masson's trichrome and Sirius red stains and hydroxyproline assay were performed for assessing collagen accumulation in the lungs of bleomycin-exposed conditional Ptx3-deficient and PTX3-neutralizing antibody (αPTX3i)-treated mice. Downstream effectors including signaling pathways and fibrotic genes were examined for assessing CD44-involved PTX3-induced fibrosis in HFL1 and primary mouse fibroblasts. PTX3 was upregulated in the lungs and plasma of bleomycin-exposed mice and correlated with disease severity and adverse outcomes in fILD patients. Decreased collagen accumulation, attenuation of alveolar fibrosis and fibrotic markers, and improved lung function were observed in bleomycin-exposed conditional Ptx3-deficient mice. PTX3 activates lung fibroblasts to differentiate towards migrative and highly collagen-expressing myofibroblasts. Lung fibroblasts with CD44 inactivation attenuated the PI3K-AKT1, NF-κB, and JNK signaling pathways and fibrotic markers. αPTX3i mimic-based therapeutic studies demonstrated abrogation of the migrative fibroblast phenotype and myofibroblast activation in vitro. Notably, αPTX3i inhibited lung fibrosis, reduced collagen deposition, increased mouse survival, and improved lung function in bleomycin-induced pulmonary fibrosis. The present study reveals new insights into the involvement of the PTX3/CD44 axis in fibrosis and suggests PTX3 as a promising therapeutic target in fILD patients. (Clin Transl Med. 2022; 12(11): e1099.) |
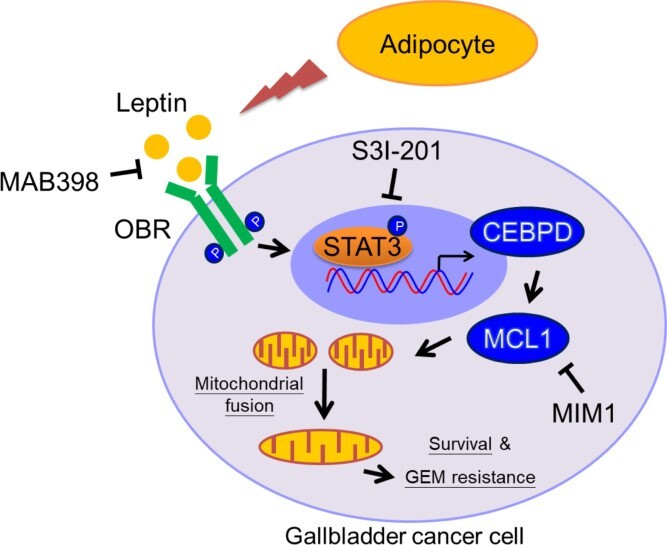 |
Obesity is a risk factor for gallbladder cancer (GBC) development, and it correlates with shorter overall survival. Leptin, derived from adipocytes, has been suggested to contribute to the growth of cancer cells; however, the detailed mechanism of leptin in GBC drug resistance remains uninvestigated. In this study, our finding that patients with GBC with a higher BMI were associated with increased GBC risks, including shortened survival, is clinically relevant. Moreover, obese NOD/SCID mice exhibited a higher circulating concentration of leptin, which is associated with GBC growth and attenuated gemcitabine efficacy. We further revealed that leptin can inhibit gemcitabine-induced GBC cell death through myeloid cell leukemia 1 (MCL1) activation. The transcription factor C/EBP δ (CEBPD) is responsive to activated STAT3 (pSTAT3) and contributes to MCL1 transcriptional activation upon leptin treatment. In addition, MCL1 mediates leptin-induced mitochondrial fusion and is associated with GBC cell survival. The findings in this study suggest the involvement of the pSTAT3/CEBPD/MCL1 axis in leptin-induced mitochondrial fusion and survival and provide a potentially new therapeutic target to improve the efficacy of gemcitabine in patients with GBC. (JCI insight, 2021; 6(15): e135438.) |

|
Name of Award |
Year of Award |
| 2024 Kwoh-ting Li Technology and Literature Lectureships Award-Honorary Scholar | 2024 |
| 2023 Kwoh-ting Li Technology and Literature Lectureships Award-Gold Award | 2023 |
| Outstanding Teachers for Industry-University Cooperation Achievements, NCKU | 2022 |
| Outstanding Research Award, Ministry of Science and Technology, MOST | 2021 |
| The 17th National Innovation Award | 2020 |
| The 16th National Innovation Award | 2019 |
| The 15th National Innovation Award | 2018 |
| First Prize of innovation and startup award in FITI | 2018 |
| Research funding award, Liver disease prevention and treatment research foundation, Taiwan | 2016 |
| The 12th National Innovation Award | 2015 |
| Achievement Award to recognize Three-times NHRI Research Grant Funding | 2014 |
| The 11th National Innovation Award | 2014 |
| Award for an outstanding achievement and presentation (Spandidos publications) | 2014 |
| Professor Chen-Yuan Lee Memorial Award, The Pharmacological Society in Taiwan | 2013 |
| Excellence in Teaching Awards, NCKU | 2013 |
| Ta-You Wu Memorial Award, NSC | 2010 |
| The Young Scientist Award, The Pharmacological Society in Taiwan | 2010 |
| Excellence in Teaching Awards, NCKU | 2010 |
| Award of Excellent Paper in Basic Medicine, Cheng-Hsing Medical Foundation | 2007 |


.svg.png)
